REPORTS
Current and Emerging Biologic Therapies for Psoriatic Arthritis
Presented by: Alice B. Gottlieb, MD, FAADProfessor of Dermatology, New York Medical College, Metropolitan Hospital, New York, NY, USA
Ixekizumab
In December of 2017, ixekizumab was approved for psoriatic arthritis. It is administered via subcutaneous injection and has a recommend dose of 160 mg (two 80 mg injections) at week 0, followed by 80 mg injections every 4 weeks. If patients have coexisting moderate-to-severe plaque psoriasis, the dosing regimen for plaque psoriasis should be employed. Patients do not need to be on concurrent methotrexate as ixekizumab can be used with or without methotrexate.
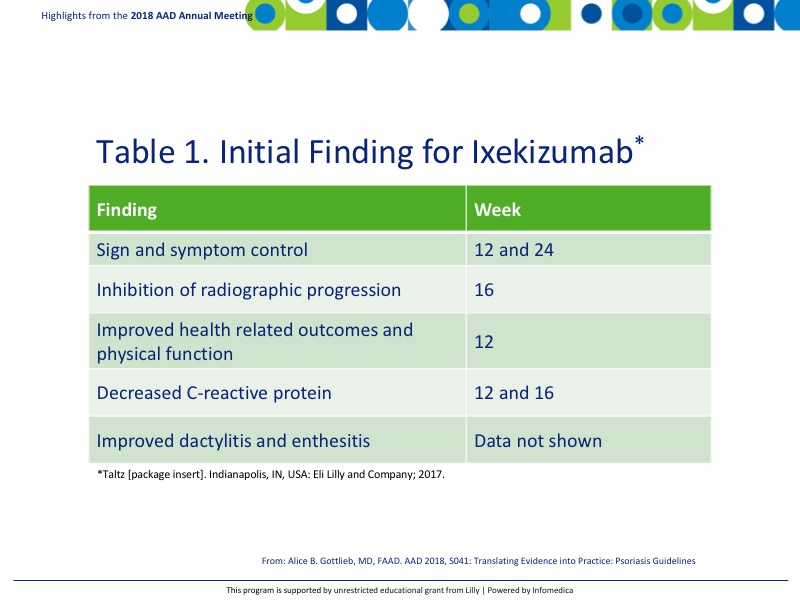
Clinical studies for ixekizumab have demonstrated significant findings that are reported in Table 1.1
Endpoints for peripheral arthritis were significant between biologic naïve patients on ixekizumab with an American College of Rheumatology 20 (ACR20) response of 57.9% and modified total sharp score (mTSS) 0.17 in the ixekizumab group compared with 31.1% and 0.49 respectively in the placebo group.2 Biologically experienced patients also demonstrated improvements with an ACR20 of 53% in the ixekizumab group compared with 19% in the placebo arm.3
Secukinumab
Secukinumab is approved for use in psoriatic arthritis. It is administered via subcutaneous injection and may or may not be administered with a loading dose. If administered with a loading dose the dosage is 150 mg every week for 4 weeks and every 4 weeks after. Without the loading dose, the dosing schedule is 150 mg every 4 weeks. If patients continue to have active psoriatic arthritis, the dosage may be increased to 300 mg. If patients have coexisting moderate-to-severe plaque psoriasis, the dosing regimen for plaque psoriasis should be employed. Secukinumab may be administered with or without methotrexate.4
Guselkumab is the only drug in current phase 2 psoriatic arthritis clinical trials.5 The multi-center, randomized, placebo-controlled study will determine the efficacy and safety of guselkumab in patients with psoriatic arthritis. The 4-part study consists of the following treatment periods:
- 6-week screening period
- 24-week double-blind guselkumab or placebo period
- Active 20-week guselkumab treatment period
- 12-week follow-up period.
The study included an early escape option where patients could switch to open-label therapy with ustekinumab 45 mg or 90 mg at weeks 16, 20, 32, and 44 for patients with <5% improvement from baseline in tender and swollen joint counts at week 16. Efficacy of guselkumab will be assessed via ACR20 response at week 24.
Abatacept is a selective T-cell co-stimulation modulator with a mechanism of action early in the inflammatory process to reduce T-cell activation.6 Initial study findings are relatively promising with an ACR20 of 39.4% and non-x-ray progression of 42.7% compared to placebo with ACR20 22.3% and 32.7%, respectively.7 PASI 75 appeared more pronounced with abatacept with 16.4% compared to 10.1% in placebo.
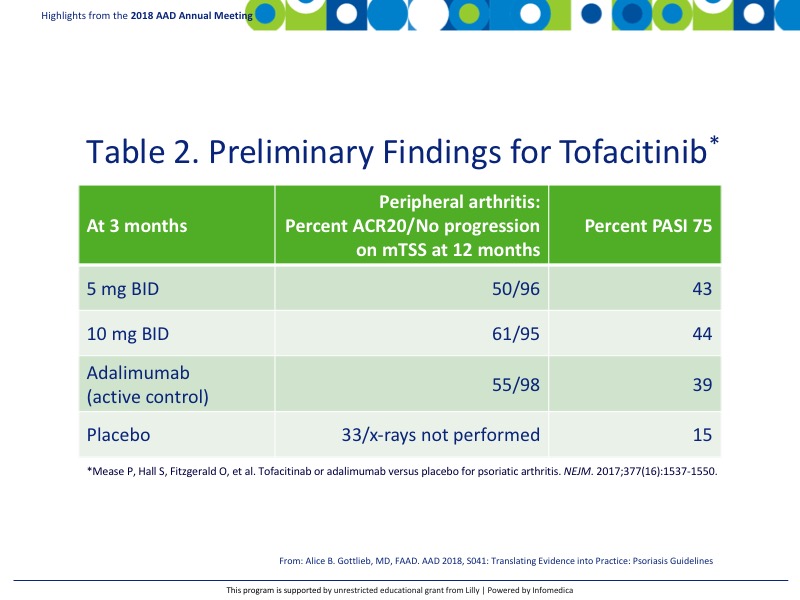
Tofacitinib is a JAK kinase inhibitor currently approved for rheumatoid arthritis. Initial studies are underway to determine its efficacy for psoriatic arthritis. Preliminary findings in biologically naïve patients are reported in Table 2.8
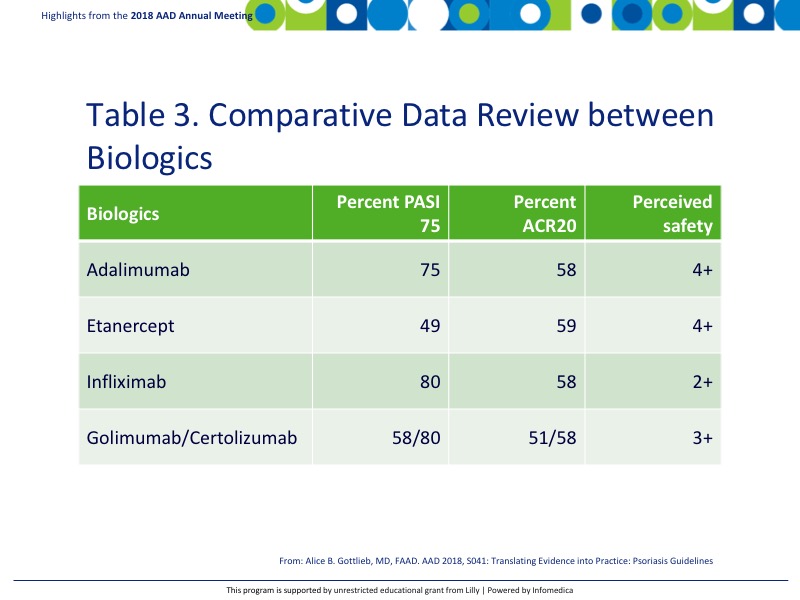
There are a handful of TNF inhibitors that are currently approved for psoriatic arthritis including adalimumab, etanercept, infliximab, golimumab, and certolizumab. Table 3 shown the comparative data review at primary endpoints PASI 75 and ACR20.9
Key messages
- There are numerous agents available for psoriasis that are under evaluation for treatment in psoriatic arthritis.
- While all of the biologics reviewed have been demonstrated to be effective in the treatment of both peripheral arthritis and skin manifestation of psoriasis, tofacitinib and abatacept did not appear to show improvement in the skin.
REFERENCES
Present disclosure: The presenter has reported that she consults, has advisory board agreements, and is involved in the speaker bureau for Janssen Inc, Celgene Corp, Bristol Myers Squibb Co, Beiersdorf, Inc, Abbvie, UCB, Novartis, Incyte, Lilly, Reddy Labs, Valeant, Dermira, Allergan, and Sun Pharmaceutical Industries.
Written by: Debbie Anderson, PhD
Reviewed by: Victor Desmond Mandel, MD
