REPORTS
Apremilast for Behçet’s Syndrome: A Phase III Randomized, Placebo-Controlled, Double-Blind Study (RELIEF)
Presented by: Yuseuf Yazici, MDClinical Associate Professor, Department of Medicine, Director, Seligman Center for Advanced Therapeutics. New York University School of Medicine, New York, NY, USA
Behçet’s Syndrome is a rare, chronic, and multi-system inflammatory disorder characterized by oral and genital ulcers, skin lesions, uveitis, arthritis, and vascular, central nervous system and gastrointestinal involvement.1,2 The condition leads to recurrent oral ulcers which can be painful and lead to a reduced quality-of-life (QOL).1,2 Apremilast is an oral phosphodiesterase-4 inhibitor that modulates several inflammatory pathways and has demonstrated efficacy in the reduction of oral ulcers in a phase 2 study (NCT00866359). However, this preliminary study was not powered to assess long-term efficacy, the effect on other manifestations of Behçet’s syndrome, or the risk of uncommon serious adverse events.1
The preliminary results from the apremilast vs placebo (RELIEF study) for Behçet’s Syndrome were presented at the American Academy of Dermatology 2018 in San Diego and are represented below.
To determine the ability apremilast to reduce the number of oral ulcers in patients with Behçet’s Syndrome over a 64-week period compared to placebo.
Type of study (NCT02307513)3
- Phase 3
- Double-blind
- Multicenter
- Placebo-controlled
- Randomized.
Patient populations
- Total number of enrolled patients: 207 (104 apremilast vs 103 placebo)
- Adults with Behçet’s syndrome meeting International study group criteria
- Oral ulcers that occurred ≥3 times in the previous 12 months
- Candidate for systemic treatment of oral ulcers
- Presence of ≥2 ulcers at screening and second visit
- No Behçet’s syndrome-related active major organ involvement in previous 12 months
- Prior treatment with ≥1 with non-biologic Behçet’s syndrome therapy.
Primary Outcome Measures
- Area Under the Curve (AUC) for number of oral ulcers.
Secondary Outcome Measures
- Change from baseline in pain of oral ulcers, by visual analogue scale (VAS)
- Behçet’s Syndrome Activity Score (BSAS) at week 12
- Behçet’s Syndrome Current Activity Index (BSCAI) at week 12
- Behçet’s Syndrome Quality-of-life (BD QOL) at week 12.
Drug/Procedures used
- Apremilast 30 mg orally twice daily for 64 weeks.
- Placebo for first 12 weeks followed by 52 weeks of 30 mg apremilast orally, twice daily.
Primary endpoints or outcomes
- AUC was statistically significant at week 12 with apremilast compared to placebo (AUC 129.5 vs 222.1, P<0.001).
- Statistically significant reduction in number of ulcers with apremilast, compared to placebo, by week 1 which was maintained through 12 weeks (P<0.05).
Secondary endpoints or outcomes
- Statistically significant reduction in rates of oral pain (change from baseline) with apremilast, compared to placebo, as early as week 1, which was maintained through 12 weeks (-40.7 vs -15.9, P<0.001).
- Statistical significance in BSAS, BDCAI, and BD QOL with apremilast compared with placebo at 12 weeks:
- BSAS -17.4 vs -5.4, P<0.0001
- BDCAI -0.9 vs -0.4, P=0.0335
- BD QOL -3.5 vs -0.5, P=0.0003.
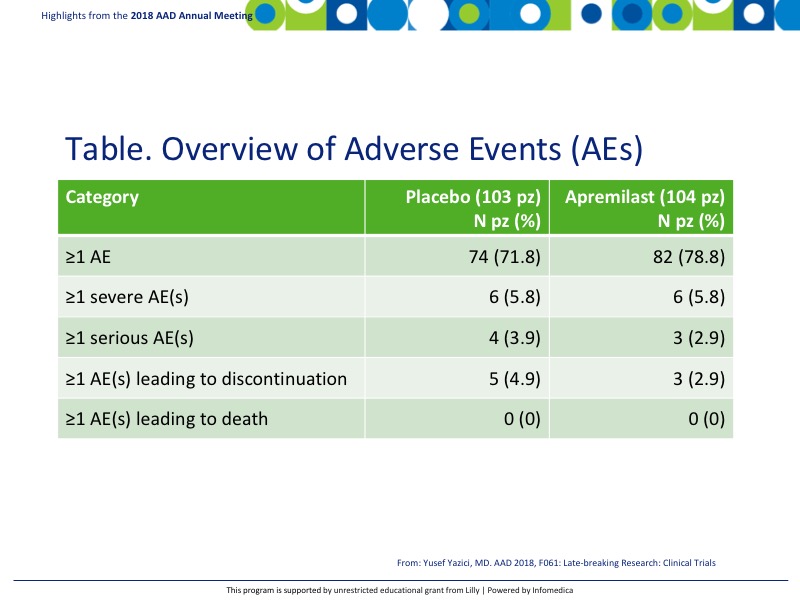
- There was good parity between adverse events (AEs) and serious AEs as can be seen in the table. No AEs led to death. The most common treatment emergent adverse events (TEAEs) that occurred in ≥5% of patients on apremilast were diarrhea 41%, nausea 19.2%, and headache 14.4%, compared to placebo 19.4%, 10.7%, and 9.7% respectively (Table).
Conclusions
- The RELIEF study demonstrated the effectiveness of apremilast in the reduction of oral ulcers in patients with Behçet’s syndrome.
- At 12 weeks, statistical significance was seen in the:
- Number and pain of oral ulcers
- Overall disease activity measures
- QOL.
- The overall AEs and TEAEs were comparable between the placebo and apremilast and demonstrated the consistent known profile of apremilast.
REFERENCES
Present disclosure: The presenter has reported that no relationships exist relevant to the contents of this presentation.
Written by: Debbie Anderson, PhD
Reviewed by: Victor Desmond Mandel, MD
