REPORTS
Efficacy and Safety of IFX-1, an Anti-C5a Monoclonal Antibody, in a Open-Label, Phase 2A Study in Patient with Severe Hidradenitis Suppurative not Eligible for Adalimumab
Presented by: Evangelos J. Giamarellos-Bourboulis, MD, PhDFourth Department of Internal Medicine, National and Kapodistrian University of Athens Medical School, Greece
There is a limited amount of knowledge known about the pathogenesis of hidradenitis suppurativa (HS). What is known is that patients with HS have an increase in complement C5a, a strong amplifier of inflammation, which is correlated with disease severity. In addition, plasma C5a also primes TNFα production in monocytes. IFX-1 is a human monoclonal antibody that specifically binds to C5a blocking its effect.
To evaluate the safety and tolerability of IFX-1 administered over 8 weeks.1
Type of study (NCT03001622)2
- Phase 2A
- Interventional
- Open-label.
Study design
- Screening:
- Duration: ≤2 weeks
- Total enrolled: 12 subjects
- Open-label Period With 800 mg IFX-1:
- 8 weeks
- Assessments: Day 1, 4, 8, 15, 22, 29, 36, 43, 50
- Follow-up period:
- Duration: 12 weeks
- Assessments: day 78, 108, 134.
Patient populations
- Age ≥ 18 years
- Diagnosis of HS for at least 1 year
- HS lesions in at least 2 distinct anatomic areas, one of which in Hurley Stage II or III
- Abscess and nodule (AN) count ≥3
- Primary or secondary failure on anti-tumor necrosis factor (anti-TNF) treatment or patient not eligible for adalimumab
- Failure of previous antimicrobial treatments.
Primary endpoint: safety
- Safety and tolerability of IFX-1 administration in patients with moderate-to-severe HS
- Adverse events (AEs).
Secondary endpoints: efficacy
- HS clinical response (HiSCR)
- HS Physician Global Assessment (PGA)
- AN count; lesion dimensions
- C5a levels.
Primary endpoints
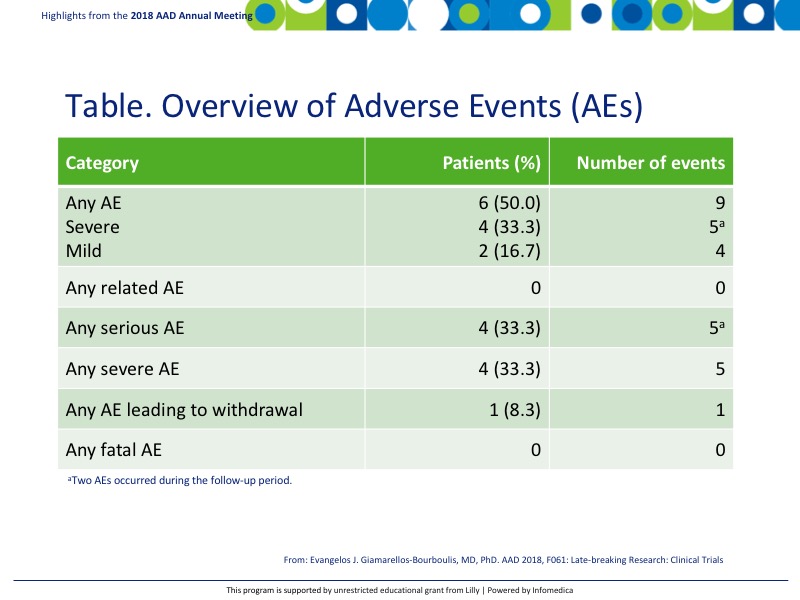
- AEs were noted in 50% (6) patients with 9 events total (Table).
- None were related to IFX-1 and no AEs were fatal.
Secondary endpoints or outcomes
- Statistically significant HiSCR was achieved by day 29 compared with day 22 (P<0.05), which was maintained through the open-label treatment period of day 50 in 9 out of 12 patients.
- The HiSCR maintained statistical significance through the follow-up period, P=0.09 on day 134 compared to day 50 in 10 out of 12 patients.
- Statistical significance was observed for AN count and lesion dimensions both at day 50 during the open-label treatment (P<0.0001 compared to day 22) and again throughout the follow-up period at day 134 (P<0.0001 compared to day 22).
- C5a levels were also significantly reduced by day 22 and maintained through day 50 (treatment phase) compared to baseline, P=0.05.
- C5a levels began to rise during the follow-up period by day 134 but were still statistically lower than original baseline values, P=0.016.
Conclusions
- IFX-1 was well-tolerated in this study and AEs were associated with HS, not to the therapy.
- The efficacy of IFX-1 demonstrated promising results with a 75% response in HiSCR at the end of treatment with an 83% HiSCR response at the end of follow-up.
REFERENCES
Present disclosure: The presenter has reported honoraria (paid to the University of Athens) from AbbVie, Biotest, Brahms GmbH, and The Medicines Company; has received compensation as a consultant for Astellas Greece, InflaRx GmbH, Germany and for XBiotech (paid to the University of Athens); and has received independent educational grants (paid to the University of Athens) from AbbVie and Sanofi. He is funded by the FrameWork 7 program HemoSpec (granted to the University of Athens) and by the Horizon2020 Marie-Curie Grant European Sepsis Academy (granted to the University of Athens).
Written by: Debbie Anderson, PhD
Reviewed by: Victor Desmond Mandel, MD
